- Most of the things are made up of compounds of carbon.
- When a carbon compound is burnt, CO2 & water are produced. The presence of CO2 can be confirmed by passing it through lime water which turns milky.
- All living structures and many non-living structures such as food, clothes, medicines, books etc. are carbon-based.
- Earth’s crust has only 0.02% carbon (as minerals like carbonates, hydrogen carbonates, petroleum, coal etc.). The atmosphere has 0.03% CO2. But carbon has immense importance.
BONDING IN CARBON – THE COVALENT BOND
Carbon compounds are poor conductors of electricity. So the bonding does not form ions. They have low melting & boiling points as compared to ionic compounds.
| Carbon Compounds | Melting point (K) | Boiling point (K) |
|---|---|---|
| Acetic acid (CH3COOH) | 290 | 391 |
| Chloroform (CHCl3) | 209 | 334 |
| Ethanol (CH3CH2OH) | 156 | 351 |
| Methane (CH4) | 90 | 111 |
- Atomic number (Z) of Carbon = 6.
- Electronic configuration = 2, 4 (1s2 2s2 2p2).
- Carbon has 4 electrons in the outermost shell. Gaining or losing 4 electrons is not possible to attain noble gas configuration because:
- Gaining 4 electrons (C4– anion) makes it difficult to hold 6 protons and 10 electrons.
- Losing 4 electrons (C4+ cation) needs high energy to leave 6 protons and two electrons.
- This problem is overcome by sharing valence electrons with other atoms of carbon or other elements. Thus both atoms attain noble gas configuration.
- The simplest molecule formed by the sharing of valence electrons is that of hydrogen (Z= 1). It has one electron in K shell and needs one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a hydrogen molecule (H2) and attain the nearest noble gas (helium - 2 electrons in K shell) configuration.
- To represent valence electrons, dots or crosses are used.
- The shared pair of electrons constitute a covalent bond between 2 hydrogen atoms. It is represented by a line.

Electron dot structure of Chlorine:

Atomic number = 17.
Electronic configuration = 2, 8, 7 (7 electrons in valence shell). It forms a diatomic molecule (Cl2).
Electron dot structure of Oxygen:
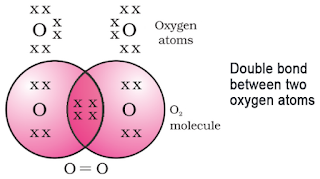
Atomic number = 8. It has 6 electrons in L shell.
It requires two more electrons to complete its octet.
So the oxygen atom shares 2 electrons with another oxygen atom forming a double bond.
Electron dot structure for water (H2O):

H – O – H
Electron dot structure of Nitrogen:

Atomic number = 7. Electronic configuration = 2, 5.
It forms a diatomic molecule (N2).
To attain an octet, each nitrogen atom in a nitrogen molecule contributes 3 electrons forming a triple bond.
Electron dot structure for methane (CH4):

- Methane is one of the simplest compounds of carbon.
- It is used as a fuel and is a major component of biogas and Compressed Natural Gas (CNG).
- Valency of Hydrogen = 1.
- Valency of Carbon = 4.
- Carbon shares these electrons with 4 hydrogen atoms to get a noble gas configuration.
- The bonds formed by sharing of an electron pair between two atoms are called covalent bonds.
- Covalently bonded molecules have strong bonds within the molecule, but intermolecular forces are weak. This gives rise to the low melting & boiling points.
- Since the electrons are shared between atoms and no charged particles are formed, covalent compounds are generally poor conductors of electricity.
Allotropes (different forms) of carbon
- E.g. Diamond, Graphite & Fullerenes.
- In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
- In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One bond is a double-bond to satisfy valency. Hexagonal arrays are placed in layers one above the other.
- Diamond & Graphite have different physical properties but same chemical properties.
- Diamond is the hardest substance. Graphite is smooth and slippery and a very good conductor of electricity.
- Synthetic diamonds can be produced by subjecting pure carbon to very high pressure and temperature. These are small but indistinguishable from natural diamonds.
- Fullerenes: The first identified one was C-60 which has carbon atoms arranged as a football. This looked like the geodesic dome designed by US architect Buckminster Fuller. So it was named fullerene.


