Metabolism of Nitrogen
Nitrogen Cycle
- Nitrogen is the most prevalent element in living organisms.
- Plants compete with microbes for limited nitrogen in soil, making nitrogen a limiting nutrient for both natural and agricultural ecosystems.
- The conversion of atmospheric nitrogen (N2 or N≡N) to ammonia is called nitrogen fixation.
- In nature, lightning and UV radiation provide energy to convert nitrogen to nitrogen oxides (NO, NO2, N2O). Industrial combustions, forest fires, automobile exhausts, and power-generating stations also produce atmospheric nitrogen oxides.
- Decomposition of organic nitrogen from dead plants and animals into ammonia is called ammonification.
- Some ammonia volatilizes and re-enters the atmosphere, but most is oxidized into nitrate by soil nitrifying bacteria (Nitrosomonas, Nitrococcus, and Nitrobacter—chemoautotrophs). These steps are called nitrification.
2NH3 + 3O2 → 2NO2− + 2H+ + 2H2O
2NO2− + O2 → 2NO3−
- Plants absorb nitrate, which is transported to leaves and reduced to ammonia, forming the amine group of amino acids.

- Nitrate in soil is also reduced to nitrogen through denitrification. It is carried out by bacteria such as Pseudomonas and Thiobacillus.
Biological Nitrogen Fixation
Biological nitrogen fixation is the reduction of N2 to NH3 by living organisms in the presence of the nitrogenase enzyme.
Only certain prokaryotic species, called N2-fixers, possess the nitrogenase enzyme and can fix N2.
Nitrogen-fixing microbes are of two types:
- Free-living: E.g., Azotobacter and Beijerinckia (aerobic), Rhodospirillum and Bacillus (anaerobic), and cyanobacteria such as Anabaena and Nostoc.
- Symbiotic: E.g., Rhizobium (aerobic).
Symbiotic Biological Nitrogen Fixation
- Legume-bacteria relationship: The most prominent example involves Rhizobium species (rod-shaped) in the roots of legumes such as alfalfa, sweet clover, sweet pea, lentils, garden pea, broad bean, and clover beans.
- The most common association on roots is as nodules.
- The microbe Frankia produces N2-fixing nodules on the roots of non-leguminous plants (e.g., Alnus).
- Rhizobium and Frankia are free-living in soil but can fix atmospheric nitrogen as symbionts.
- The central part of a nodule is red or pink due to leguminous haemoglobin (leg-haemoglobin).
Principal stages in nodule formation:
- Rhizobia multiply and colonize the surroundings of roots, attaching to epidermal and root hair cells.
- Root hairs curl, and bacteria invade the root hair.
- An infection thread carries bacteria into the root cortex, initiating nodule formation.
- Bacteria are released into cells, leading to the differentiation of specialized nitrogen-fixing cells.
- The nodule establishes a direct vascular connection with the host for nutrient exchange.

- Nodules contain the nitrogenase enzyme and leg-haemoglobin.
- Nitrogenase (a Mo-Fe protein) catalyzes the conversion of N2 to NH3, the first stable product of N2 fixation.

N2 + 8e− + 8H+ + 16ATP → 2NH3 + H2 + 16ADP + 16Pi
- Ammonia synthesis requires high energy input (8 ATP per NH3), obtained from the respiration of host cells.
- Nitrogenase is highly sensitive to molecular oxygen, requiring anaerobic conditions. Leg-haemoglobin acts as an oxygen scavenger to protect it.
- Rhizobia are aerobic under free-living conditions (when nitrogenase is not operational) but become anaerobic during N2-fixing events to protect nitrogenase.
Fate of Ammonia
- At physiological pH, NH3 is protonated to form NH4+ (ammonium) ion. Most plants can assimilate nitrate and NH4+, but NH4+ is toxic and cannot accumulate in plants.
- NH4+ is used to synthesize amino acids in two ways:
a. Reductive Amination:
Ammonia reacts with α-ketoglutaric acid to form glutamic acid.
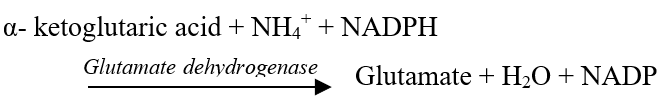
b. Transamination:
The amino group (NH2) is transferred from one amino acid to the keto group of a keto acid in the presence of a transaminase enzyme. Glutamic acid is the main amino acid from which NH2 is transferred to form other amino acids.

- Asparagine and glutamine are important amides in plants, forming structural parts of proteins. They are derived from aspartic acid and glutamic acid by adding another amino group, replacing the hydroxyl part of the acid with an NH2 radical.
- Since amides contain more nitrogen than amino acids, they are transported via xylem vessels. Additionally, nodules of some plants (e.g., soybean) export fixed nitrogen as ureides, which have a high nitrogen-to-carbon ratio.

best notes
ReplyDeleteBest notes ever
ReplyDelete