Enzymes
- Enzymes are biological catalysts which influence the speed of biochemical reactions.
- All enzymes are proteins but all proteins are not enzymes.
- Enzymes are specific. i.e., each enzyme has its own substrate.
- Ribozymes: Nucleic acids (RNA) that behave like enzymes.
- Enzymes form tertiary structure (3D) with some crevices (pockets) called ‘active site’ into which the substrate fits.
Chemical Reactions
Chemical compounds undergo two types of changes:
- Physical change: A change in shape or state of matter without breaking bonds. E.g., ice melts into water, water becomes vapour.
- Chemical change (chemical reaction): In this, bonds are broken and new bonds are formed. It may be organic or inorganic reaction. E.g.
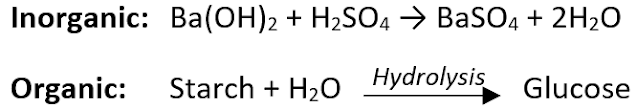
Rate of a physical or chemical process = Amount of product formed per unit time. i.e., δp/δt.
Rate is called velocity if the direction is specified.
Rates of physical and chemical processes are influenced by factors such as temperature. Generally, rate doubles or decreases by half for every 10°C change in either direction.
Rate of enzyme catalysed reactions is very high. E.g., Carbonic anhydrase is the fastest enzyme. It accelerates the following reaction 10 million times.

In the absence of enzyme, only 200 molecules of H2CO3 are formed in an hour. In the presence of carbonic anhydrase, about 600,000 molecules are formed per second.
In a metabolic pathway, each step is catalysed by different enzymes.
E.g., In glycolysis [Glucose (C6H12O6) → 2 Pyruvic acid (C3H4O3)], ten different enzymes take part.
Nature of Enzyme Action (Catalytic Cycle)
Enzyme acts with substrate like a lock & key model action.
It includes the following steps:
- The substrate binds to the active site of enzyme (E+S).
- This induces some changes in enzyme so that the substrate is tightly bound with active site of enzyme to form enzyme-substrate complex (ES).
- The active site breaks chemical bonds of substrate to form enzyme-product complex (EP).
- The enzyme releases the products and the free enzyme is ready to bind to other molecules of the substrate (E+P).

This pathway goes through some unstable transition state structures.
How do Enzymes Speed up a Chemical Reaction? (Concept of Activation Energy)
- Activation energy is the additional energy required to start a chemical reaction.
- In an exothermic or endothermic reaction, the substrate must go through a much higher energy state. It is called transition state energy. Therefore, activation energy is the difference between average energy of substrate and transition state energy.
- If the product (P) is at a lower energy level than the substrate (S), the reaction is an exothermic reaction (spontaneous reaction). It requires no energy (by heating) to form the product.
- In a biochemical reaction, enzymes lower the activation energy. As a result, speed of the reaction increases.

Factors Affecting Enzyme Activity
a) Temperature and pH
Enzymes show highest activity at optimum temperature & pH. Activity declines below and above optimum value.
At low temperature, enzyme temporarily inactive.
At high temperature, enzymes destroy because proteins are denatured by heat.

Inorganic catalysts work at high temperature & pressure. But enzymes get damaged at high temperature (> 40°C).
Thermophilic organisms have enzymes which are stable at high temperature (up to 80-90°C).
b) Concentration of Substrate

With the increase in substrate concentration, the velocity of enzyme action rises at first and reaches a maximum velocity (Vmax). This is not exceeded by further rise in concentration because enzyme molecules are fewer than the substrate molecules i.e., No free enzyme molecules to bind with additional substrate molecules.
c) Presence of Inhibitor
The binding of specific chemicals (inhibitor) shuts off the enzyme activity. This is called inhibition.
The inhibitor closely similar to the substrate is called competitive inhibitor. It competes with the substrate for the binding site of the enzyme. As a result, the substrate cannot bind and the enzyme action declines. E.g., Malonate is similar to the substrate succinate. So, it inhibits succinic dehydrogenase in the following reaction.

Competitive inhibitors are used to control bacterial pathogens.
Classification and Nomenclature of Enzymes
- Oxido-reductases / Dehydrogenases: Catalyze oxido-reduction b/w two substrates.
S reduced + S’ oxidized → S oxidized + S’ reduced
- Transferases: Catalyze transfer of a group (other than hydrogen).
S-G + S’ → S’-G + S
- Hydrolases: Catalyze hydrolysis of ester, ether, peptide, glycosidic, C-C, C-halide or P-N bonds.
- Lyases: Catalyze removal of groups by mechanisms other than hydrolysis leaving double bonds.
X-C-C-Y → X-Y + C=C
- Isomerases: Catalyze inter-conversion of optical geometric or positional isomers.
- Ligases: Catalyze the linking of 2 compounds together. E.g., enzymes catalyzing joining of bonds like C-O, C-S, C-N, P-O etc.
Co-factors
These are non-protein constituents bound to the enzyme to make the enzyme catalytically active.
Apo-enzyme: Protein portion of the enzyme.
Co-factor + Apoenzyme = Holoenzyme.
When the co-factor is removed from the enzyme, its catalytic activity is lost.
Co-factors are 3 types:
- Prosthetic group: Organic. Tightly bound to apoenzyme. E.g., Haem. It is a part of the active site of peroxidase and catalase. These enzymes catalyze breakdown of H2O2 to water & O2.
- Co-enzymes: Organic. Transient binding to apoenzyme. Many co-enzymes contain vitamins. E.g., nicotinamide adenine dinucleotide (NAD) and NADP contain niacin.
- Metal ions: They form co-ordination bonds with side chains at active site and one or more co-ordination bonds with the substrate. E.g., Zn is a cofactor for Carboxypeptidase.

Thank you
ReplyDelete