Biomacromolecules (Macromolecules)
These are biomolecules having molecular weight greater than 1000 Da. They include:
- Proteins
- Polysaccharides
- Nucleic acids
Their molecular weight is 10,000 Da and above.
Acid insoluble fraction (macromolecular fraction) includes macromolecules from cytoplasm and organelles.
Lipid is not strictly a macromolecule as its molecular weight does not exceed 800 Da. But it comes under acid insoluble fraction because many lipids are arranged into structures like cell membranes. When a tissue is grinded, cell membranes are broken and form water insoluble vesicles. They cannot be filtered along acid soluble fraction.
| Average Composition of Cells | |
|---|---|
| Water | 70-90% |
| Protein | 10-15% |
| Carbohydrates | 3% |
| Lipids | 2% |
| Nucleic acids | 5-7% |
| Ions | 1% |
1. Proteins
- Proteins are heteropolymer of amino acids.
- They are polypeptides, i.e., linear chains of amino acids linked by peptide bonds.
- Peptide bond is formed when –COOH group of one amino acid reacts with NH2 group of next amino acid by releasing a molecule of water (dehydration).
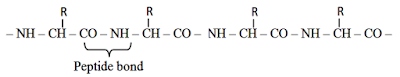
Functions of Proteins
- For growth and tissue repair.
- Transport nutrients across cell membranes. E.g., GLUT-4 enables glucose transport into cell.
- Acts as intercellular ground substance. E.g., collagen.
- Acts as antibodies to fight infectious organisms.
- Acts as receptors. E.g., receptors of smell, taste, hormones.
- Some are hormones (e.g., Insulin), enzymes (e.g., trypsin), pigments (e.g., hemoglobin) etc.
Most abundant protein in animal world: Collagen.
Most abundant protein in the biosphere: Ribulose bisphosphate carboxylase - oxygenase (RuBisCO).
Structural Levels of Protein
- Primary structure: It describes the sequence of amino acids, i.e., the positional information in a protein.
- Secondary structure: Here, one or more polypeptide chains are folded in the form of a helix. It has only right-handed helices. E.g., Keratin, Fibroin (silk fibre).
- Tertiary structure: Here, helical polypeptide chain is further folded like a hollow woolen ball. It gives 3-D view. Tertiary structure is necessary for many biological activities of proteins. E.g., Myoglobin, enzymes.
- Quaternary structure: Here, more than one polypeptide chains form tertiary structure and each chain functions as subunits of protein. E.g., Haemoglobin. It has 4 subunits (2 α subunits and 2 β subunits).

2. Polysaccharides (Complex Carbohydrates)
These are polymers of sugars (monosaccharides). E.g.:
- Starch (homopolymer of glucose)
- Cellulose (homopolymer of glucose)
- Glycogen (homopolymer of glucose)
- Inulin (homopolymer of fructose)
There are complex polysaccharides formed of amino-sugars (e.g., glucosamine, N-acetyl galactosamine etc.).
Chitin is the homopolymer of N-acetyl glucosamine. It is seen in exoskeleton of arthropods and fungal cell wall.
Glycosidic bond in polysaccharides: It is the bond formed when individual monosaccharides are linked between 2 carbon atoms by dehydration.
Starch forms helical secondary structure. It can hold iodine molecules in the helical portion giving blue colour.
Cellulose has no complex helices and so cannot hold iodine.
Diagrammatic Representation of a Portion of Glycogen
3. Nucleic Acids (DNA & RNA)
Nucleic acids are heteropolymer of nucleotides. i.e., many nucleotides are linked to form polynucleotide.
Nucleic acids are 2 types: DNA (Deoxyribonucleic acid) and RNA (Ribonucleic acid).
Secondary Structure of DNA (Watson - Crick Double Helix Model)
- There are more than a dozen forms of DNA such as A, B, C, D, E, Z etc.
- DNA consists of 2 polynucleotide strands arranged antiparallelly as a double helix.
- In DNA, a nucleotide consists of nitrogen base, deoxyribose sugar and phosphate group.
- Backbone (strands) of DNA is formed by the sugar-phosphate-sugar chain.
- Steps are formed of Nitrogen base pairs.
- Nitrogen bases include Adenine (A), Guanine (G), Thymine (T) and Cytosine (C). Uracil absent.
- A pairs with T (A=T) by 2 hydrogen bonds.
- G pairs with C (G≡C) by 3 hydrogen bonds.
- A phosphate molecule links the 3’-carbon of the sugar of one nucleotide to the 5’-carbon of the sugar of the next nucleotide. The bond between the phosphate and –OH group of sugar is an ester bond. As there is one such ester bond on either side, it is called phosphodiester bond.
- The bond between sugar and nitrogen base is called N-glycosidic bond.

In B-DNA
- One full turn of helical strand has 10 steps (10 base pairs).
- Length of one full turn = 34 Å (i.e. 3.4 Å for each step).
- At each step, the strand turns 360 (3600 for a full turn).
