TYPES OF CHEMICAL REACTIONS
- In a chemical reaction, atoms of one element do not change into atoms of another element. Nor do atoms disappear from the mixture or appear from elsewhere.
- Chemical reactions involve breaking and making of bonds between atoms.
1. Combination Reactions
- These are the reactions in which a product is formed by combining two or more reactants.
- E.g. Take some calcium oxide (quick lime) in a beaker. Slowly add water to this. Beaker becomes hot. Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing heat.

- Slaked lime solution is used for whitewashing. Calcium hydroxide reacts slowly with CO2 in air to form a thin layer of calcium carbonate (CaCO3) after 2-3 days of whitewashing giving a shiny finish to the walls. Marble is also CaCO3.

Other examples of combination reactions:
- Burning of coal:
- Formation of water from H2 and O2:
Reactions in which heat is released along with formation of products are called exothermic chemical reactions.
Other examples of exothermic reactions:
- Burning of natural gas:
- Respiration:
- Decomposition of vegetable matter into compost.
- Burning magnesium to form magnesium oxide.
(Glucose) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy
2. Decomposition Reactions
These are the reactions in which single reactant breaks down to give simpler products.
Examples:
- Take 2 g ferrous sulphate crystals in a dry boiling tube. Heat it over flame. Green colour of ferrous sulphate is changed with characteristic odour of burning sulphur. When heated, ferrous sulphate (FeSO4.7H2O) lose water. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).


- Decomposition of CaCO3 to calcium oxide (CaO) & CO2 on heating is used in industries. CaO has many uses (e.g. manufacture of cement). When a decomposition reaction is carried out by heating, it is called thermal decomposition.

Other examples of thermal decomposition:
Example 1
- Take 2 g lead nitrate powder in a boiling tube.
- Hold the boiling tube and heat it over a flame. Brown fumes of nitrogen dioxide (NO2) are emitted.


Example 2
- At the base of a plastic mug, drill two holes and fit rubber stoppers.
- Insert carbon electrodes in rubber stoppers. Connect electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed.
- Add few drops of dil. H2SO4 to the water.
- Take two test tubes filled with water and invert them over the electrodes.
- Switch on the current. Bubbles are formed at electrodes by displacing water in test tubes.
- At cathode (-ve electrode), hydrogen gas is collected. At anode (+ve electrode), oxygen is collected. At cathode, double amount of gas is collected as compared to anode because during the break down of water, 2H molecule is released with 1 oxygen molecule.

When we bring a burning candle to the gas at cathode, it burns immediately. But gas at anode does not burn.
Example 3
- Take 2 g silver chloride in a china dish. Place this in sunlight for some time. Its white colour turns grey. This is due to the decomposition of silver chloride into silver and chlorine by light.

- Silver bromide also behaves in the same way.
- Decomposition reactions of silver chloride and silver bromide are used in black & white photography.
- Decomposition reactions require energy (heat, light or electricity) to break down the reactants.
- Reactions in which energy is absorbed are called endothermic reactions. E.g.
- Take 2 g barium hydroxide in a test tube. Mix 1 g of ammonium chloride. The following reaction occurs.
- It is an endothermic reaction because it absorbs heat from environment. So bottom of the test tube becomes cool.
3. Displacement Reactions
- These are the reactions in which a more reactive element displaces a less reactive element from its compound.
Examples:
- Take 3 iron nails cleaned by rubbing with sand paper. Take two test tubes A and B. Add 10 mL copper sulphate (CuSO4) solution in each test tube. Immerse two iron nails in CuSO4 in test tube B. Keep one iron nail aside for comparison. After 20 minutes, take out the iron nails. They become brownish in colour. Blue colour of CuSO4 solution fades. This is due to the following chemical reaction:
- Here, iron has been displaced by copper from the CuSO4 solution.



Other examples of displacement reactions:

Zinc and lead are more reactive elements than copper. They displace copper from its compounds.
4. Double Displacement Reactions
These are the reactions in which there is an exchange of ions between the reactants.
Examples:
- Take 3 mL sodium sulphate solution in a test tube. In another test tube, take 3 mL barium chloride solution. Mix the two solutions.
- Reaction between lead nitrate & potassium iodide to form a yellow precipitate of lead iodide.
A white substance (BaSO4) is formed by the reaction of SO42– and Ba2+. This water insoluble substance is called precipitate.
The other product (sodium chloride) remains in solution.
Any reaction that produces a precipitate is called precipitation reaction.


5. Oxidation and Reduction
- Oxidation is the gain of oxygen or loss of hydrogen during a reaction. The substance is said to be oxidised.
- Reduction is the loss of oxygen or gain of hydrogen during a reaction. The substance is said to be reduced.
Examples:
- Burning of a magnesium ribbon in air (oxygen) gives magnesium oxide. Here magnesium is oxidised.
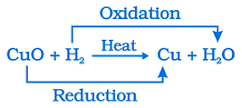
- Heat a china dish containing about 1 g copper powder. The surface of copper powder becomes coated with black copper(II) oxide. This is because oxygen is added to copper and copper oxide is formed.


If hydrogen gas is passed over the heated CuO, the black coating turns brown as the reverse reaction takes place and copper is obtained.

During this reaction, copper(II) oxide loses oxygen and is reduced. The hydrogen gains oxygen and is oxidised.
When one reactant gets oxidised and the other gets reduced in a reaction, it is called oxidation-reduction reaction (redox reaction).
Other examples of redox reactions:
- Carbon is oxidised to CO and ZnO is reduced to Zn.
- HCl is oxidised to Cl2 whereas MnO2 is reduced to MnCl2.
EFFECTS OF OXIDATION REACTIONS IN EVERYDAY LIFE
1. Corrosion
- It is a process by which a metal is attacked by substances such as moisture, acids, etc. E.g. black coating on silver, green coating on copper.
- Iron articles get coated with a reddish-brown powder when left for some time. This is called rusting of iron.
- Corrosion causes damage to car bodies, bridges, iron railings, ships and to all objects made of metals.
2. Rancidity
- When fats and oils are oxidised, they become rancid and their smell and taste change.
- Oxidation and rancidity can be prevented by:
- Adding antioxidants (substances which prevent oxidation) to foods containing fats and oil.
- Keeping food in air tight containers.
- Flushing bags of chips with gas such as nitrogen.
